
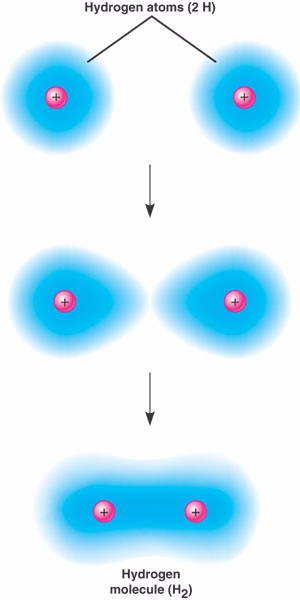
 Covalent bond.
Covalent bond.
Each hydrogen atom has a single electron (represented as a cloud)
in its valence shell - shell #1.
The two valence electrons be shared in a strong covalent bond,
forming an H2 molecule.
In the resulting molecule, the shared pair of electrons enable each atom to complete its valence
shell.
Two pairs of valence electrons can also be shared to form a double covalent bond.