
 Water properties.
Water properties.
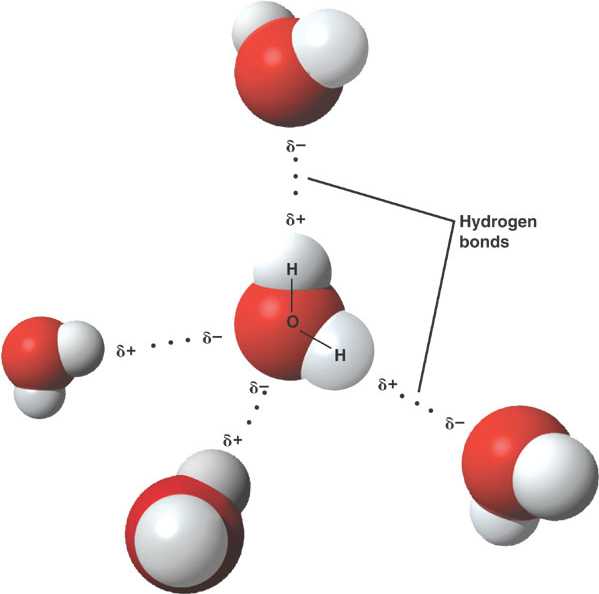
A water molecule is held together by strong, polar covalent bonds between oxygen and hydrogen atoms.
The partially charged regions of a polar water molecule are attracted to oppositely charged parts of neighboring molecules.
Each molecule can form weak hydrogen bonds to multiple partners, conferring water unique properties.